|
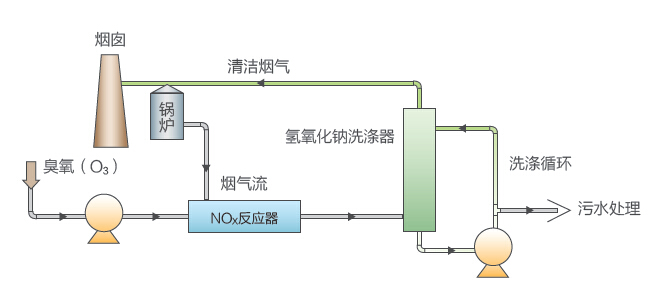
Oxidized NO
occupies more than 95% in smoke and gas into high-value NO2, NO3 or N2O5. NO
does not dissolve in water, but NO2, NO3 or N2O5 reacts with water and produces
HNO3 and dissolves greatly. Combining with Alkali absorption (desulfurization
system), NO2, NO3 or N2O5 can remove 80% of sulphur.
 |